Abstract
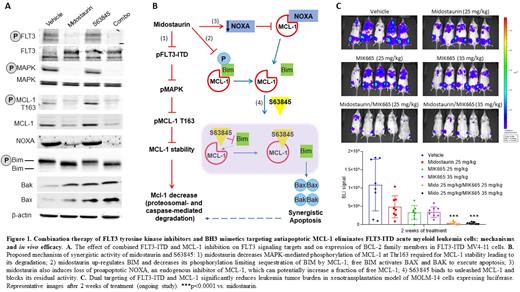
Activating FLT3 internal tandem duplication (FLT3-ITD) mutations, present in ~30% of AML patients, are associated with poor prognosis. Given that efficacy of FLT3 inhibitors used as monotherapy is limited due to development of secondary FLT3 mutations or activation of alternative survival pathways, new combinatorial strategies are needed to improve outcome in FLT3-ITD AML patients. Constitutive activation of mutated FLT3 often leads to elevated expression of MCL-1, an anti-apoptotic member of the BCL-2 family. Upregulation of MCL-1 underlies the resistance of AML to initially successful targeted therapies, including Bcl-2 inhibition with venetoclax, placing MCL-1 as an attractive target for combinatorial therapies. We have recently shown for the first time that a novel MCL-1 inhibitor S63845 elicited synergistic activity with FLT3 inhibitors AC220, sorafenib and with a multi-kinase inhibitor midostaurin at nanomolar doses in pre-clinical in vitro models of FLT3-ITD AML, including cells resistant to venetoclax (Skwarska A, et al. Cancer Res 2019;79 Suppl 13, Abstract#342). Here, we further explored the therapeutic potential and mechanisms-of-action of dual targeting of FLT3-ITD and MCL-1 in vitro and in vivo. S63845 and midostaurin co-treatment significantly increased apoptosis in FLT3-ITD cells with caspase-3 activation and PARP cleavage within 6 hours of treatment. Analysis of drug combinations using Bliss independence model revealed strong synergistic effect of S63845/midostaurin combination in murine isogenic Ba/F3-FLT3-ITD, Ba/F3-FLT3-ITD-D835Y and Ba/F3-FLT3-ITD-F691L dual mutants with minimal effect in Ba/F3-wt FLT3 cells, suggesting that inhibition of FLT3-ITD could mechanistically contribute to the synergy between midostaurin and S63845. Midostaurin monotherapy caused de-phosphorylation of FLT3-ITD and its downstream targets STAT5, AKT and MAPK, known to upregulate MCL-1 expression and stability (Fig 1A). Consequently, midostaurin significantly reduced Mcl-1 T163 phosphorylation and protein levels exclusively in FLT3-ITD cells. Midostaurin also decreased inhibitory phosphorylation of GSK3, suggesting the involvement of ubiquitin/proteasome axis in MCL-1 degradation. Additionally, pre-treatment with pan-caspase inhibitor zVAD-fmk partially reversed MCL-1 downregulation, implying contribution of caspase-mediated degradation to overall decrease of MCL-1 after midostaurin treatment. While midostaurin alone enhanced degradation of MCL-1, it did not fully deplete the pool of MCL-1. Surprisingly, midostaurin also induced the loss of proapoptotic NOXA, an endogenous inhibitor of MCL-1, which can potentially increase a fraction of free MCL-1. These results provide a strong rationale for targeting residual MCL-1 with selective MCL-1 inhibitor S63845 with the goal to facilitate deeper therapeutic responses in FLT3-ITD AML. Mechanistically, midostaurin increased expression of apoptosis activator BIM and blocked BIM phosphorylation required for its inhibitory sequestration by MCL-1. BIM shRNA knockdown cells were less sensitive to midostaurin/S63845 combination. Similarly, CRISPR/Cas9-mediated knock-out of BAK, a BIM-activated executor of apoptosis, resulted in reduced efficacy of tested combination. These results indicate that activation of BIM/BAX axis has a functional role in the response of AML cells to dual FLT3-ITD and MCL-1 inhibition (Fig 1B). To validate the efficacy of combination in vivo, we used xenotransplantation model of MOLM-14 cells expressing luciferase. Mice were treated for 3 weeks with low doses of midostaurin (25 mg/kg/5 days per week) and with MIK665, structurally optimized version of S63845 Mcl-1 inhibitor with improved pharmacokinetics in mice (Halilovic E, et al. Cancer Res 2019;79 Suppl 13, Abstract#4477). Administration of MIK665 at 25 mg/kg or 35 mg/kg IV 2 days per week in combination with oral midostaurin resulted in a marked delay in the progression of MOLM-14 cell line-derived xenograft and significantly reduced leukemia tumor burden (Fig 1C). The long-term effect of combination on mice survival is currently being tested and will be reported.
Altogether, our results indicate that efficacy of FLT3-ITD inhibitors can be enhanced through combination with low doses BH3 mimetics targeting MCL-1 and provide a rationale for the clinical evaluation of such combinations in FLT3 mutant AML patients.
Skwarska: Halilovich E, Wang Y, Morris E, Konopleva M, Skwarska A.: Patents & Royalties: Combination of a MCL-1 inhibitor and midostaurin, uses and pharmaceutical composition thereof.. Halilovic: Novartis: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Mistry: Novartis: Current Employment, Current holder of individual stocks in a privately-held company. Derréal: Servier: Current Employment. Banquet: Servier: Current Employment. Daver: Genentech: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Novimmune: Research Funding; Glycomimetics: Research Funding; Trovagene: Consultancy, Research Funding; FATE Therapeutics: Research Funding; Astellas: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Hanmi: Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Sevier: Consultancy, Research Funding; Novartis: Consultancy; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding. Andreeff: Medicxi: Consultancy; ONO Pharmaceuticals: Research Funding; AstraZeneca: Research Funding; Reata, Aptose, Eutropics, SentiBio; Chimerix, Oncolyze: Current holder of individual stocks in a privately-held company; Karyopharm: Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Glycomimetics: Consultancy; Novartis, Cancer UK; Leukemia & Lymphoma Society (LLS), German Research Council; NCI-RDCRN (Rare Disease Clin Network), CLL Foundation; Novartis: Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy; Aptose: Consultancy; Oxford Biomedica UK: Research Funding; Amgen: Research Funding; Breast Cancer Research Foundation: Research Funding; Senti-Bio: Consultancy. Wei: Novartis, Astellas, Pfizer, MacroGenics, AbbVie, Genentech, Servier, Celgene, Amgen, AstraZeneca, Janssen: Honoraria; Novartis, Celgene, AbbVie, Servier, AstraZeneca, and Amgen: Research Funding; Walter and Eliza Hall Institute: Ended employment in the past 24 months. Konopleva: Cellectis: Other: grant support; Stemline Therapeutics: Research Funding; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; AstraZeneca: Other: grant support, Research Funding; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Ascentage: Other: grant support, Research Funding; Agios: Other: grant support, Research Funding; Sanofi: Other: grant support, Research Funding; Forty Seven: Other: grant support, Research Funding; Ablynx: Other: grant support, Research Funding; KisoJi: Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; Calithera: Other: grant support, Research Funding; Rafael Pharmaceuticals: Other: grant support, Research Funding; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding.